View our GSA Schedule 65 II-A: 36F79720D0194
01
Our Partners
Just a few of our partners. If there is a product you cannot find on our website please let us know. If we don’t already have it available, we will get it for you.

core values
- Honesty
- integrity
- respect
- Discipline
02
Government
Frontline Services Inc. is a Service Disabled Veteran Owned Small Business (SDVOSB) that connects medical device manufacturers with the Federal Marketplace. We offer a full-service medical contract and billing solution as well as deliver advanced medical technologies to the federal government. Our products meet the unique needs of military surgeons and patients and are clinically proven to improve treatment outcomes. As fellow military veterans, we support you and your patients.
View our GSA Schedule 65 II-A: 36F79720D0194
DLA DAPA: SP0200-21-H-0021
03
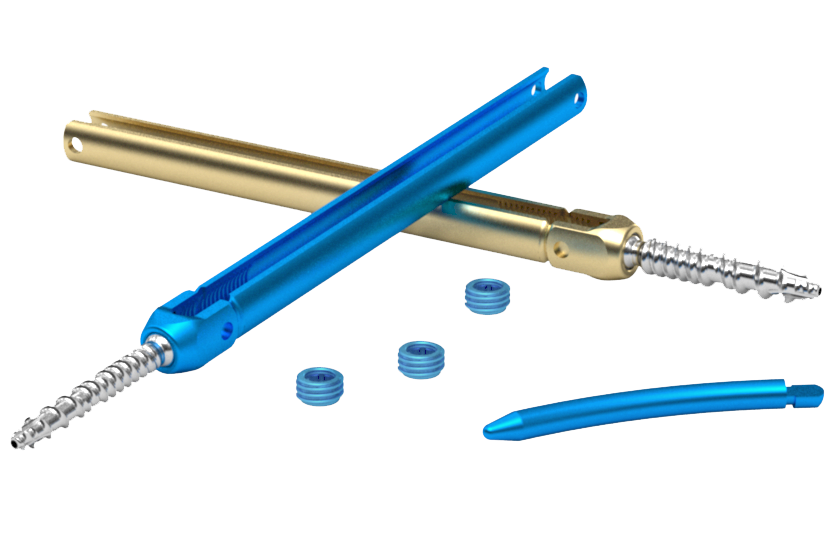
Just a few of our most popular products.
our products
F.A.Q.
Frequently Asked
We’ve put together some commonly asked questions to give you more information about the products and services we offer.
If you have a question that you cannot find the answer to, please contact us at info@flservicesinc.com.
Yes. All implant products provided through Frontline Services have received FDA 510k. FDA documentation remain current and on file at our corporate office.
Most implants provided are available in validated, sterile packaging, with only some exceptions. Products which are not sterile packaged through our vendors can be sterilized according to manufacturers’ prescribed protocols.
Yes. Working collaboratively with the surgeon and our vendor partners, FDA-compliant custom instrumentation can be designed and manufacturer to the surgeon’s preference, with usually very short lead times.
Yes. From pre-op to post-op phases of each episode of care, Frontline Services plays its role to ensure facility-compliant services are provided to successfully support the surgeons pre-, intra- and post-operative surgical support requirements.
Yes. All Frontline Services team members are certified by its vendor partners, and are qualified to support surgical cases.
Yes. All Frontline Services team members maintain current hospital credentialing, ie Symplr, and others, as necessary.
The Synthetic based products are designed and produced in the manufactures lab to satisfy the design rationale that the manufacturer sets forth. These products contain mainly bTCP (beta-Tricalcium Phosphate) and/or HA (Hydroxyapatite) which are compounds found in bone.
The Tissue based products are derived from human tissue that has been processed from human cadaveric bone which has been donated. This is a very regulated environment and must meet specific standards for human tissue to be accepted and donated.
The tissue products that Frontline has chosen to represent has taken an extra step in safety by following all the AATB guidelines and added in sterilizing the final product while most other manufacturer’s only process aseptically (clean, but not sterile).
Frontline has researched and found that the biologics that we represents has a unique carrier that has the smallest % by volume of carrier and a carrier that is designed to thicken and maintain the “Active Ingredient” at the desired site for the longest possible time without compromising the surgeons objective.
Contact Us
Let's Get in Touch